Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 1, 2011

Growing commercial demand for therapeutic monoclonal antibodies (MAbs) has increased the need for efficient and cost-effective manufacturing processes. The development of cell cultures with very high expression levels is one clear example. over recent years, the antibody titers of mammalian cell culture have risen dramatically, and today we often see titers of 5–10 g/L.
Expression levels as high as 15 g/L or greater have even been reported. Chromatography media (resins) that can capture these high-titer antibodies are therefore essential for translating this potential gain into a smooth downstream purification process free from costly bottlenecks. Similarly, media with effective purification performance over a long working lifetime will help ensure good and predictable manufacturing economy.
We describe quantitative studies made on MabSelect SuRe* LX, a recent GE Healthcare protein A affinity medium that fulfills both of the above criteria by offering high dynamic binding capacities for MAb capture as well as extended long-term chromatographic performance over hundreds of purification and cleaning-in-place (CIP) cycles. Modern MAb Purification Strategies
The large-scale purification of MAbs usually consists of two or three chromatographic steps. Protein A is the affinity chromatography ligand of choice for initial capture, and its high selectivity gives excellent purity (typically >99%) in one step. The GE Healthcare MabSelect* family of affinity media is widely used by commercial manufacturers of MAbs.
MabSelect SuRe LX is based the same rigid high-flow agarose matrix and alkali-stabilized ligand as the related MabSelect SuRe medium. However, its attributes of higher dynamic binding capacity (DBC) and extended lifetime performance offer greater efficiency and economic benefits to large-scale manufacturing. Dynamic Binding Capacity Studies
The performance of MabSelect SuRe LX during MAb capture was investigated by measuring its DBC (10% breakthrough) at different residence times and feed concentrations. The resulting data were compared with MabSelect SuRe. In addition, process economy parameters — specifically medium productivity and utilization during manufacturing — were evaluated for both media. Polyclonal IgG and clarified CHO cell culture supernatants containing various monoclonal antibodies were used in the study.
Initial results showed that DBC for monoclonal IgG1 and polyclonal IgG increased with residence time for MabSelect SuRe LX at residence times between 1 and 10 minutes (Figure 1, TOP). Comparing data for both media (Figure 1, BOTTOM) showed that DBC is almost equivalent at short residence times (up to ~3 minutes). Above 3 minutes, however, MabSelect SuRe LX shows significantly higher DBC.
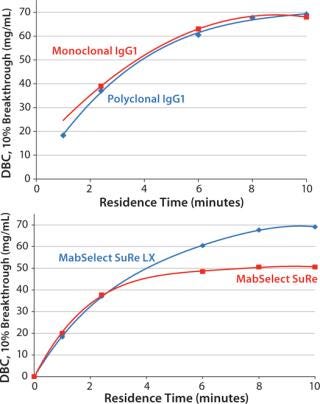
Figure 1: ()
When the DBC for MabSelect SuRe LX was determined with clarified CHO cell culture at three MAb concentrations and three residence times between 2.4 and 10 minutes, it was observed that MAb concentration did not have any significant influence on DBC. As expected, however, DBC did increase with increased residence time. In addition, determining DBC for MabSelect SuRe LX and MabSelect SuRe at 6 minutes residence time for seven different MAbs revealed that the DBC was 20% to 46% higher for MabSelect SuRe LX. Lifetime Performance Study with Repeated CIP
A general lifetime study was also performed on the long-term chromatographic performance of MabSelect SuRe lX during repeated purification cycles that use 0.1 M or 0.5 M NaoH for CIP. Parameters measured included DBC (DBC, 10% breakthrough) at different residence times, yield of antibody, and levels of leached protein A and host cell proteins (HCP). Using 0.1 M NaoH as CIP agent for 100 cycles, the dynamic capacity of MabSelect SuRe LX decreased only slightly after 10 cycles and then remained constant throughout the study (Figure 2). Yield was also high (>97%) and stable throughout. in addition, no significant changes in leached protein a or HCP levels over the 100 cycles were observed.
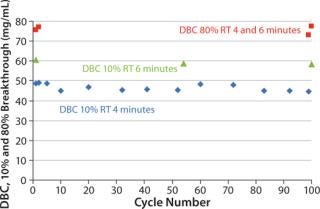
Figure 2: ()
To further compare MabSelect SuRe LX with MabSelect SuRe, additional lifetime studies were performed using CIP solutions with 0.1 M or 0.5 M NaoH. The results shown in Figure 3 once again demonstrate the excellent stability of MabSelect SuRe LX over repeated purification cycles, and confirm its superior DBC compared with MabSelect SuRe. Following CIP with 0.5 M NaoH, for example, 80% of DBC remained after 150 cycles for MabSelect SuRe LX compared with 125 cycles for MabSelect SuRe. this indicates about a 20% longer lifetime for MabSelect SuRe LX.
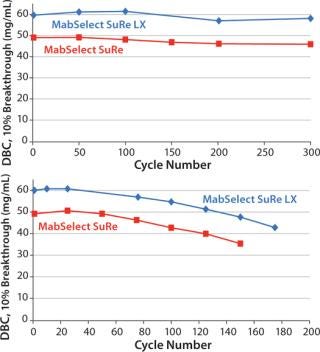
Figure 3: ()
Summary
The high binding capacity of MabSelect SuRe LX, combined with the proven mechanical stability of its high-flow matrix, translates into better processing productivity compared with MabSelect SuRe. Related gains can also be expected from a reduction in the quantity of medium and number of cycles required to purify a given mass of antibody, as well as in overall buffer consumption for the purification step.
Long-term performance data confirm that the alkali-stabilized protein a ligand of MabSelect SuRe LX is very stable and demonstrate that it withstands repeated CIP with 0.1 M to 0.5 M NaoH with at least a 20% higher DBC (at 6 minutes residence time) plus an increased lifetime of around 20%,
MabSelect SuRe LX should purify significantly more antibody than MabSelect SuRe over its working life. Further information can be found in the following application notes:Lifetime Performance Study of MabSelect SuRe LX During Repeated Cleaning-in-Place (GE Healthcare 28-9872-9
6, edition AA, 2011) and Dynamic Binding Capacity Study on MabSelect SuRe LX for Capturing High-Titer Monoclonal Antibodies (GE Healthcare 28-9875-25, edition AA, 2011).
GE, imagination at work and GE monogram are trademarks of General Electric Company. *MabSelect and MabSelect SuRe are trademarks of GE Healthcare companies. © 2011 General Electric Company – All rights reserved. First published July 2011.
About the Author
Author Details
Anders Ljunglöf is a scientist, Hans J Johansson is a staff scientist, and John Joseph is a scientist in the R&D department at GE Healthcare Life Sciences, Björkgatan 30, SE-751 84 Uppsala, Sweden; [email protected]; www.gelifesciences.com; www.gelifesciences.com.
You May Also Like