Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 1, 2011

Selecting a CMOis critical to biotech biologic drug development. A key factor for selecting a CMOis experience. How do you measure experience? It can be measured in many ways, metrics include
Number of GMP processes developed by the CMO
Number of projects involving your strain
Number of projects involving your product type
GMP production success rate
Number of GMP lots produced.
Eurogentec’s Biologics Division is specialized in the manufacturing of biopharmaceuticals from microbial sources such as bacteria, yeast, and biosafety level 2 organisms. Eurogentec manufactures in accordance with GMP since 1994.
Host Cell Experience — Microbial Experts
Escherichia coli
Pichia pastoris
Hansenula polymorpha
Saccharomyces cerevisiae
Numerous BSL-2 organisms.
Chemistry Experience — Conjugation Experts
Maleimide
Glutaraldehyde
Other chemistries available.
Product Family Experience
Recombinant proteins
Plasmid DNA
Protein–PEG conjugations
Protein–protein conjugations
Protein–peptide conjugations.
GMP Infrastructure
Multiproduct facility
Separate HVAC systems
Three GMP fermentation suites (80 L, 150 L, 500 L)
Two GMP purification suites
One GMP sterile filtration suite.
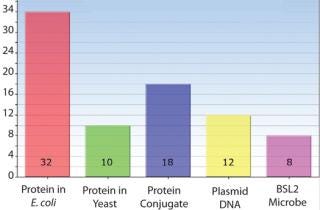
Figure 1: ()
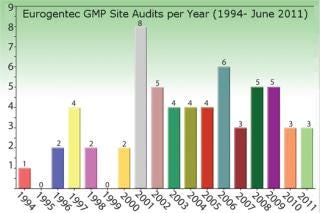
Figure 2: ()
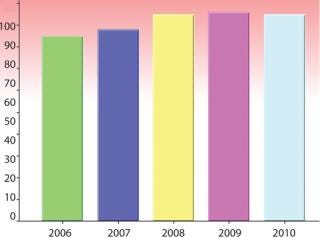
Figure 3: ()
Process Development Strategies
FastTrack — quick to clinic
OptiTrack — robustness built in.
About the Author
Author Details
Dr. Pascal Bolon is biologics sales and marketing manager at Eurogentec SA, Rue du Bois Saint-Jean 5, 4102 Seraing, Belgium; 32-4-366-6116; [email protected].
You May Also Like