Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 1, 2011

Both industrial and research scale protein purification require the development of superior procedures for increasing recovery yields. This places an emphasis on maintaining proteins in fully reduced states to solubilize inclusion bodies, while increasing the recovery of active proteins through improved protein-folding technologies. These concerns have a common theme: the ability to maintain the disulfide bonds in the protein in a sufficiently reduced state so as to facilitate correct folding while simultaneously avoiding protein aggregate formation. Dithiothreitol (DTT) is still a choice reagent to correctly achieve this delicate balance (1).
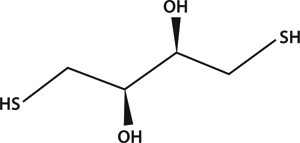
DTT, or Cleland’s reagent, is a low–molecular-weight thiol that reduces disulfide bonds quantitatively and maintains monothiols in the reduced state. These properties enable many varied applications including in vivo studies of protein processing, in situ solubilization of proteins, protein folding, protein purification, induction of genes involved in signal transduction, synthetic oligonucleotide synthesis, and protection of thiol groups during organic synthesis (1). Protein Purification
Over-expression of proteins in Escherichia coli commonly results in the production of insoluble, misfolded protein aggregates known as inclusion bodies. To obtain an active protein from an inclusion body, an inactive protein must be solubilized and correctly refolded into its native active form, often through oxidative processes. DTT is often used in the solubilization and refolding of inclusion bodies because it reduces disulfide bonds and maintains them in the reduced state. During solubilization, the protein is denatured using strong denaturants such as urea or guanidium chloride at high concentrations. This renders any disulfide bonds present more accessible for reduction. DTT is quite often used in this step because it is a strong reductant (1).
BioVectra CGMP DTT Quality Attributes
Manufacturing work instructions (BPR’s) and associated QC forms are controlled and issued to manufacturing by Quality Assurance (QA)
Verifications are required for all critical processing steps such as raw material charges, temperature profiles, etc.
All raw materials and starting materials are subject to statistical sampling for QC testing prior to use in production. Vendors of these raw materials are approved, and materials are qualified.
Analytical methods for final product testing are validated. Total impurities do not exceed 1%, and residual solvent analysis is completed.
QA releases product upon completion of a batch record review.
The process is subject to an annual product review by manufacturing and QA
The product is subject to an annual stability program
Protein folding is initiated by transferring the protein from denaturing conditions into renaturing conditions. In proteins containing cysteine residues, there is a greater likelihood of aggregates forming by disulfide scrambling, a process in which the disulfide bonds form incorrectly during protein folding. In such systems, the folding buffer must be supplemented with a redox system comprising both reduced and oxidized forms of low-molecular-weight thiols. The thiol-disulfide exchange system increases the rate and yield of protein renaturation/oxidation by reshuffling any incorrect disulfide bonds formed. Agents usually used in oxido-shuffling systems include reduced and oxidized glutathione (GSH/GSSG), cysteine/cystine, cysteamine/cystamine, dttred/oxidized glutathione, and dithioerythritol (dte)/oxidized glutathione.
Rothwarf and Scheraga have used the redox couple of DTTOX and DTTRED to study the folding pathway of bovine pancreatic ribonuclease A. They noticed that the regeneration pathway was different when using DTT compared with using oxidized and reduced glutathione. A much simpler folding pathway made it possible to examine the process clearly without resorting to a series of complex equations (1).
Need for cGMP Bioprocess Reagents
Considering such applications for DTT and the close product contact inherent in the reagent’s use, concern has increased about product contact and the quality designation of material. Because BioVectra is the world’s primary manufacturer of DTT, it has been able to offer a catalogue of cGMP-grade material. A Model to Move Forward
This is BioVectra’s model for cGMP bioprocessing reagents, and BioVectra is currently working with additional manufacturing partners to offer bioprocessing reagents as cGMP grade. Its next cGMP material is another reducing agent, TCEP (Tris-Cyanoethylphospine).
BioVectra is an experienced cGMP manufacturer of active pharmaceutical ingredients (APIs) and select final drug products. Both its internal materials and product packaging processes as well as its external product distribution system are established to provide the level of controls necessary in handling products under controlled distribution conditions.

About the Author
Author Details
Heather Howatt is director of worldwide sales and marketing for BioVectra Inc., 11 Aviation Avenue, Charlottetown, PE Canada C1E 0A1; 1-866-883-2872, ext. 6236; [email protected].
1.) O’Handley, D. 2002. Dithiothreitol (DTT) in Protein Processing and Purification. Am. Bio. Lab. 20:22.
You May Also Like