Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
February 1, 2009
This paper describes how RFID (Radio Frequency Identification) technologies can be implemented into single-use systems to generate electronic records for both bag manufacturers and bag end-users. RFID technology will enable the user to both read and write all relevant product and process information directly onto the single-use bag, providing instantaneous data recall. The tag can also provide the user with immediate access to the bag’s original part number, lot number, date of manufacture, expiration date and other critical item-level data that is normally contained in the product certificate of quality.
Gamma-stable RFID tags attached to disposable bag systems, writing and reading software and handheld data reader systems can now be used to offer customers a unique paperless record, improve traceability, retrieve release documentation and leverage the capabilities of RFID in their facilities. The system presented in this paper utilizes Radio Frequency Identification technology to write identification information as well as lot-specific information including part number, lot number, description, manufacture and expiration dates. Tag-in-Bag RFID technologies provide wireless error-proof recording of critical product-related information in a faster and more reliable manner compared with conventional paper, labels and barcode systems.
RFID Technology and Applications
Basic principles of RFID: Radio Frequency Identification (RFID) is an automatic identification technology that uses radio waves to communicate. Fixed or mobile readers communicate with transponders, commonly called tags, to identify items, collect data and, in many implementations, write data into the tag’s memory. There are several types of RFID systems available (Low Frequency, LF; High Frequency, HF; Ultra High Frequency, UHF; and Microwave, 2.45 GHz), each with its own benefits. For the purposes of this article, we will focus on passive High Frequency (HF) RFID. A passive tag is one that does not contain a battery to power the device. Power for the processor is provided inductively through the electromagnetic field generated by the reader’s antenna. The result is a tag that has a shelf-life of many years, or decades, without loss of data or functionality. Commonly referred to as vicinity or near-field RFID, HF tags are ideal for applications that require item-level identification, part discrimination and the ability to write unique information to individual items. The processor built into the tag responds to commands issued by the reader to exchange information such as the Globally Unique Identifier (GUID) of the tag or the contents of the memory. This processor is also capable of anticollision, allowing the reader to communicate with many tags simultaneously. Read rates of up to 40 tags per second are achievable.
Existing biotech applications: Flexible connections used in the manufacture of pharmaceutical and biotech products have been the subject of increased scrutiny in recent years. Lost production because of hose failures, contamination or failed audits caused by the inability to document the cleaning processes, age, usage history or origin of hoses has become more common. Silicone-based hoses, in particular, experience degradation due to sterilization processes such as autoclaving. Hose integrity is more directly related to the cumulative effect of these cleaning methods than the age of the hose. Monitoring and evaluating whether a hose is fit for use can reduce the occurrence of hose failures. Other risks associated with flexible connections are difficulty in identifying product-specific hoses, difficulty in calculating useful life, downtime owing to replacement lead times, lost or misfiled documentation, and difficulty in correlating batch records with hose logbooks. The use of RFID-enabled hoses and readers to identify and record use, maintenance and wear-related events in a central database can provide significant benefits. The database can be queried to perform life cycle analysis on the hoses, calculate useful life, minimize the incidence of failure and move away from a calendar replacement methodology. This life cycle analysis will allow production facilities to maximize the life of under-utilized hoses and replace over-utilized hoses in a timely manner. Production personnel can access hose-related information from anywhere on the network through a simple, secure interface, including retrieving the manufacturer’s pedigree information across the Internet. Audits and investigations can be conducted faster and more efficiently as the database contains the complete audit trail for the hose and does not require logbooks to be gathered and reviewed.
Filtration and purification from pilot through to large-scale production requires the specific application of filtration technology. Flow rate and bioburden reduction are important for economic benefits and the protection of downstream processing equipment. Confirmation of the appropriate cartridge selection can easily be achieved with RFID-enabled filters. Additionally, transcription errors derived from the manual entry of cartridge data can be eliminated. Not only can this information be recorded in the batch record or transferred to an electronic logbook, it can be used to automatically enter the integrity test specification into an integrity testing device and track the life cycle of a filter. Diaphragm replacement and preventive maintenance can be more efficiently conducted utilizing RFID-enabled valves. Product contact information can be recorded to minimize the number of diaphragm replacements caused by host changes. Scheduled and unscheduled maintenance can be recorded on the valve and uploaded to the database. Maintenance procedures, installation instructions, drain angles, drawings and certifications can also be retrieved from the database.
Other technologies (barcodes): Barcodes have traditionally been used to offer a paperless record of product-related information. Barcodes offer less flexibility compared with RFID tags as they are limited to a small amount of characters and information and cannot be written by end-users (Table 1).
Table 1: Differences between barcodes and RFID tags.

Table 1: Differences between barcodes and RFID tags. ()
Advantages of Gamma-Stable RFID Tags
Gamma-stable RFID tags have the unique ability to retain their functionality and data after exposure to the ionizing radiation commonly used in single-use bioprocessing equipment. The ability to survive routinely acceptable gamma sterilization doses allows identification and the encoding of pedigree information during manufacturing without the logistical challenges of returning the product to the manufacturing facility to add RFID labelling to the product. Manufacturers can provide RFID-enabled bioprocessing equipment without incurring the significant additional costs associated with redesigning their distribution process. High Frequency RFID operating at 13.56 mHz is a science and technology frequency, and most countries have accepted it as an unlicensed frequency for these applications. This allows for the standardization of hardware and reduces the complexity of the support system for RFID implementations. Tags have 2 Kb of memory (2048 bytes), allowing for a significant amount of part-specific data and associated data to be recorded (Figures 1 and 2).
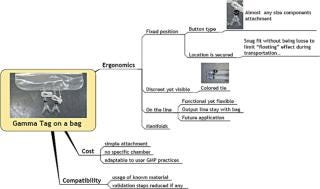
Figure 1: ()

Figure 2: ()
Data Integrity
Although all RFID tags, regardless of frequency, have a Globally Unique Identifier (GUID), most require a closed-loop data infrastructure (requesting related information from a database based on the unique ID) to translate the GUID into meaningful data. High Frequency tags have two unique features that allow for a high level of data integrity: an on-board memory and a locking mechanism to secure that data. The on-board memory of HF RFID tags provides a convenient means of storing item-specific information directly on the RFID tag, allowing for the retrieval of item-specific data without the need of a database or a connection to the manufacturer’s information. This locking mechanism provides a means of ensuring that the data will be preserved throughout the life of the item. It is a non-reversible operation performed at the chip level, allowing a manufacturer to write item-specific data to the tag and trust that it will be unaltered. Data integrity is ensured because pedigree information, such as the part or serial number, can be written to the tag memory and locked permanently.
Quality Control
Process control is a fundamental priority in pharmaceutical and biotech manufacturing. RFID enables manufacturing facilities to provide item-level status information on each bag system. As each process is completed, the event relevant to that bag (incoming inspection, release for production, media fill, QA release) can be recorded on the individual container. These activities are recorded without the application of labels or writing directly on the bag, thus eliminating the risk of adhesive or ink volatiles permeating the bag chamber. Additionally, bags in storage retain their information regardless of whether or not they get moved around.
ISO standards ISO 15693
One of the main reasons for choosing High Frequency RFID (HF) for single-use bag systems is that it is a global standard. Being a science and technology frequency, it does not require licensing and country specific hardware to implement.
Implementation of RFID Tags on Single-Use Assemblies
Making RFID tag use achievable by end-users depends on the bag manufacturer’s capability to implement the technology in its own operations. Lot information is retrieved from the ERP system, written to individual tags, verified, locked to prevent manipulation, and the transaction is recorded in the database. Gamma-stable tags are written with product lot information during our production process. Tag Writer software has been developed to write and read RFID tags with information associated with single-use assemblies, such as the following:
Product part number
Product description
Lot number
Manufacturing date
Expiry date.
Used from a PC by authorized staff only, this application can retrieve product lot information stored in the central ERP database and write tags in a fast and reliable way. The tags are attached by means of a cable tie to the disposable assembly, which is then packaged and gamma sterilized. The Tag Writer is interfaced with the ERP used at the bag assembly/manufacturing sites to automatically get product lot information from the work order number. Each time a tag is written using the Tag Writer, the following information is recorded in a log:
Date/hour of the operation
User name
The operation (Read or Write)
The status of the operation (OK or Error)
The ID of the tag
The recorded information (part number, description, lot, manufacturing date, expiry date).
This information is stored in a password-protected database available to Tag Writer administrators for auditing purposes (Figure 3and Table 2).
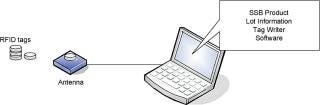
Figure 3: ()
Table 2: RFID Tag record structure for lot information.
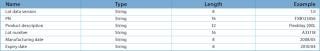
Table 2: RFID Tag record structure for lot information. ()
Tag Writer runs under the Windows XP user interface. At the start-up, the user is required to login using his/her Windows password. The screenshot in Figure 4 displays the main screen. The use of Tag Writer is limited to users who belong to one of the following groups: “Tag Writer Admin” — read/write tags and access to the log database for audit “Tag Writer” — read and write tags “Lot Reader” — read tags only. The left hand side of the screen is only available to users belonging to the “Tag Writer Admin” and “Tag Writer” groups. The right hand side is available to users belonging to any one of the three Tag Writer users groups. The “Set Values” button activates a function that allows set values to be stored in the tag. These are automatically retrieved from the work order information. The Tag Writer software is designed to help users successively record a set of tags. Therefore, once the recorded values have been set, the default button activated by the “Enter” key should be “Write Tag.” After each successful “Write Tag” action, the “Read Tag” action is activated. The “Read Tag” button background is set to green if the data has been permanently written on the tag successfully (Figure 5).
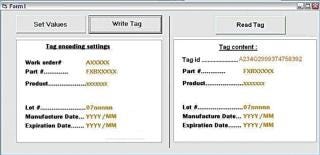
Figure 4: ()
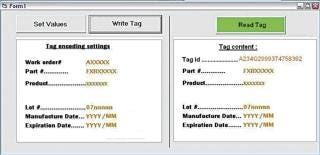
Figure 5: ()
Tag Writer is able to write product lot information on a tag and record the action in the log in less than 1 second. The default occurrence of tags should be less than 1/10,000. The source code of Tag Writer must be available for audit and debugging. Log data is stored in a separate database protected by a password. The log database is encrypted and must be regularly backed-up by the local administrator.
End-User Implementation
End-users receive single-use bags that are already equipped with a written tag as specified above. The tag is fixed at the extremity of one of the tubes to facilitate access and the reading of the written product lot-related information. The Process Equipment Tracking (PET) software has been designed to run on a handheld device. It reads the tag and records the written information in the device memory. The handheld device is then connected to a docking station and the PET software, running on a PC, can download the tag information from the device into the customer database, which stores audit trail information and can track documents associated with bag assembly (such as lot information and certificate of release) and to the customer bioprocess. The PET software can be customized according to the customer’s needs; for instance, it can read and display the information on the handheld device before transferring it to the PC workstation as well (Figure 6).
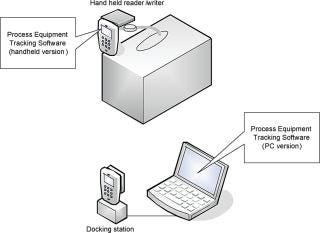
Figure 6: ()

Figure 7: ()
Conclusion and Outlook
The implementation of gamma-stable RFID tags in single-use processes is now a reality and end-users of single-use bag products can actually use this innovative technology to provide secure, fast and paperless records of product part numbers, product descriptions, lot numbers, manufacturing and expiry dates. This is only a first milestone in exploiting the full potential of gamma-stable RFID tags. In the short-term, RFID tags will encode complete product lot release information. End-users will then be able to retrieve the complete Certificate of Quality for their single-use bags by logging on to an internal database via a specific and confidential code embedded onto the tag. The part number and lot number information retrieved with the handheld device will be combined to form a URL that could be displayed in Internet Explorer to view the associated release certificate PDF document from a website. End-users can also use the remaining memory on the prewritten RFID tag that is attached to the bags to write and store drug product lot and process data information, and store this information in an audit trail database. Product- and process-specific information can include all the major life cycle events of a bag at the customer’s site:
Incoming Inspection data
Customer specific expiration date
Quarantine and release of empty bag for use
Dates and operator for each event
Filled bag release: date, operator, product, product lot number filled
Process information: flow rate, volume in bag, pH, conductivity, formulation, etc.
Bulk drug substance manufacturers could then record lot-specific information on shipping bags before delivering to the final fill provider, who will verify and transfer it to the associated packaging more efficiently and accurately. The integration of sensors with RFID will open up even more new opportunities for process control and monitoring. They could be combined in the future with disposable sensors to automatically record critical process parameters. For example, temperature, pH, conductivity, flow rate, weights, volume and other readings could all be recorded to ensure the proper storage and handling of information needed to monitor the processing, mixing and storage of biopharmaceutical products.
You May Also Like