Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
July 1, 2008

Figure 1.
As a leading provider of process and analytical solutions for the biotechnology and pharmaceutical industries, Applied Biosystems offers an extensive portfolio of tools to help optimize every stage of biopharmaceutical production — from characterization to purification to pharmaceutical manufacturing. These solutions provide pharmaceutical manufacturers with the tools they require to maximize throughput and yield and ensure product quality.
Purification
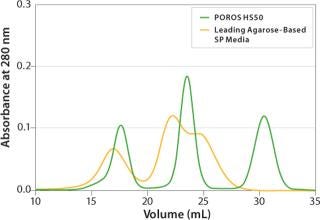
High Performance on a Large Scale: Applied Biosystems POROS Perfusion Chromatography products are today’s best-performing chromatographic media for process-scale bioseparations. These rigid, robust particles enable high-resolution separations with two to three times the throughput of conventional fast-flow gels. They are easier to handle and pack, and they offer outstanding cleanability. All POROS process-scale media products are backed by full regulatory support information in a drug Master File. POROS media are used in the manufacture of numerous FDA-approved biotherapeutics and many products in various stages of clinical production. For more information, visit www.appliedbiosystems.com/poros.
Pharmaceutical Manufacturing
The Need for Product Quality — Impurity Analysis in Biopharmaceutical Manufacturing: Product safety and quality are paramount in pharmaceutical manufacturing. Recent incidents point to increasing contamination problems that expose pharmaceutical companies to escalating financial risk as well as mounting political and legal pressures for regulatory control.
For biopharmaceutical manufacturing, product quality can be ensured through in-process testing using genotypic-based detection methods. Accurate, same-day results can help companies increase productivity and reduce risk through rapid, routine detection of contaminants from cell banks, cell culture, purification, filling, and freeze-drying.

SEQ™ Rapid Molecular Methods for pharmaceutical manufacturing include premium genotypic-based detection and identification systems focused on product safety and product quality, along with 24/7 technical support from a proven technology provider with over 25 years of leadership in life science research. For more information, visit www.microseq.com, or contact [email protected].
MicroSEQ® Microbial Detection Systems: Using industry-standard real-time polymerase chain reaction (RT-PCR), these detection systems provide unparalleled sensitivity and specificity in detecting mycoplasma and residual DNA.
MicroSEQ® Microbial Identification System: Using a genotypic approach based on rRNA genes, this system has been proven 100% accurate and 100% reproducible in identifying thousands of bacteria and fungi in pharmaceutical environments. The complete solution includes sample preparation, integrated instruments, optimized reagent kits, IQ/OQ Service, implementation, and training programs.
You May Also Like