- Sponsored Content
- Facility Design/Engineering
- Single Use
Designing Single-Use Facilities for Biomanufacturing Expansion
Sponsored by Sartorius
Minimizing a facility footprint while maximizing manufacturing capacity is essential to staying agile, productive, and cost-effective — all of which are key elements to competing in a dynamic business landscape.
To achieve such efficiency at commercial scale, bioprocessing facility design should be tailored to each organization’s specific needs. During scale-up, tailor-designed facility planning is critical to streamlined manufacturing of high-quality products. The size and layout of a space can otherwise become a limiting factor for long-term productivity, revenue, profit, and regulatory approval. Therefore, early consideration of operations is essential.
A number of factors will shape planning decisions: Modalities, operations, and budgets can encourage biomanufacturers to incorporate single-use (SU) processing and process-intensification strategies to meet target yields, costs, and timelines. Such next-generation manufacturing approaches will introduce specific layout needs to an overall design strategy. Here, we discuss how to prioritize business drivers for efficient design of a commercial-scale manufacturing facility.
Top Considerations
Critical factors that guide facility design include business, process, facility, and technology drivers.
Business: One primary differentiator of facility size, layout, and capacity is whether a biomanufacturer plans to produce a single product or multiple products. A multiproduct facility serving a global market will require significantly greater flexibility and throughput.
Process: Manufacturers then must consider how to set up a production approach to maximize productivity and minimize cross-contamination risks.
Different modalities will have different dosing requirements and, therefore, batch sizes. Volume and throughput are critical factors when a company begins sizing different manufacturing lines and deciding room allocations in multiproduct facilities. For example, lentiviruses (LVs) typically are used for cell therapies and are manufactured in small volumes (up to 50–200-L scale), whereas adenoassociated viruses (AAVs) typically used for gene therapies or vaccines require up to 2,000-L batch sizes.
The need to manufacture multiple products also will affect how a facility operates; bioprocess scientists must decide whether their processes can run concurrently or whether a campaign approach is more appropriate. Different manufacturing lines can be set up for different modalities and volumes, or a single production suite can be created to manufacture all products.
Good manufacturing practice (GMP) guidelines recommend that multiproduct facilities use a campaign-based approach, segregating production by time rather than by space (1). That can prevent two distinct modalities from operating in the same suite concurrently, eliminating chances for cross-contamination.
Facility: A facility design should meet the needs of an intended number of products, modalities, and production scales. Some organizations might be constrained by the size and layout of existing facilities, whereas companies developing new facilities have the opportunity to purpose-build them to support unique needs.
A segregation approach is essential to creating a safe and effective production suite while ensuring that manufacturing remains streamlined and productive. Biomanufacturers must not underestimate additional regulatory and containment requirements for scaling up to commercial production — e.g., by misjudging the extent of GMP and non-GMP areas.
Segregation is particularly important for multiproduct facilities (in which manufacturers must prevent occurrence and demonstrate the absence of batch-to-batch contamination), for processes reliant on living organisms, and for processes that expose operators to risks. If a manufacturer is planning to set up microbial, cell culture, and viral-based processes in a single facility, the company will have to consider the design of the process rooms, waste management strategy, and biosafety level (BSL) boundaries for viral-process areas (which also can include separate HVAC systems).
For LV and AAV production, it is crucial to consider different segregation concepts driven by different BSL requirements as well as differing batch sizes. Typically, AAV production requires BSL-1 precautions, whereas LV manufacture requires BSL-2 or BSL‑2+ facilities. The differences in scales and BSL requirements can require use of different suites and unidirectional flows for materials and personnel.
Implementing SU systems in a modular design can help improve flexibility and limit downtime, especially in multiproduct facilities. Such modern layouts operate with limited fixed equipment, and processes are segregated by use of automation and closed systems. The more process closure can be ensured with SU technologies, the less reliance is needed on segregation.
A modular approach is particularly suitable for production of cell and gene therapies, which tend to be produced on much smaller scales and for fewer individuals. Such products need less initial investment and a smaller facility footprint than in a traditional cleanroom facility, speeding up construction and reducing operating costs.
Technology: Platform and equipment selection depend on the specific nature of an intended product, including batch size and process parameters.
SU Technologies: SU technologies are known to provide ease of use, flexibility, consistency, and low upfront investment, among other benefits. SU is now widely established across the bioprocess industry, with an estimated ≥85% of precommercial manufacturing now carried out using SU systems (2). During facility design, biomanufacturers must consider how they will implement SU technologies, whether by selecting end-to-end SU platforms or identifying opportunities for SU solutions in a hybrid facility.
Process Intensification: Implementing intensification strategies reduces costs, accelerates production, improves yields, and enhances flexibility (3). The benefits of intensified production strategies must be considered in the context of facility planning because they can influence equipment and fluid management strategies. For instance, N – 1 perfusion uses existing equipment, requiring minimal changes to an established infrastructure to achieve 60% better productivity with just a 10% increase in a USP and media preparation footprint. (4).
Supporting Expansion Steps
Expertise in conceptual design, cost modeling, technology, and regulatory know-how can help ease the transition to commercial scale while limiting capital investment, reducing operating costs, and maximizing facility throughput.
Turning Ideas into Reality
Sartorius offers bioprocess consulting services with a process-first approach, tailoring end-to-end SU manufacturing solutions from concept to realization. Our Integrated Solutions team collaborates with biomanufacturers and GMP facility-design partners to provide a thorough service, which includes a unique implementation methodology for your bioprocess using SU technologies wherever possible and hybrid solutions as appropriate for your type and scale of process.
Using a conceptual design study, we build a picture of your business needs, including modalities, process requirements, level of automation, room layout design, and cost of goods. We then work with you to verify optimum mass balance and design a process flow diagram. This includes selecting suitable equipment of the size required and a room layout that best accommodates your process and incorporates material and personnel flow, automation, and integration concepts (including process monitoring and data analytics, Figure 1). Modeling different process scenarios allows us to identify bottlenecks and optimize facility setups before engineering and execution.
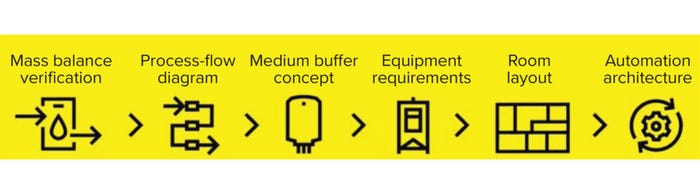
Figure 1: Conceptual design approach through Sartorius Integrated Solutions.
Partnering with a supplier that has the knowledge and tools to design the most efficient facility to fit your needs will minimize your risks and simplify your journey toward a productive and regulatory-compliant facility (see the “How Sartorius Supports Your Capacity Expansion” box).
Our bioprocess consultants help you establish a facility that fits your process needs rather than refit your process into an ill-fitting facility. Early considerations of key facility design variables together with adoption of SU technologies and intensification strategies can help you design a flexible modular facility for the dynamic biopharmaceutical landscape. Learn more about how Sartorius can help you create a reliable manufacturing facility at https://www.sartorius.com/en/services/bioprocess-development-engineering.
References
1 EU GMP Annex 2: Manufacture of Biological Medicinal Products for Human Use. EudraLex, 2019: https://www.gmp-compliance.org/guidelines/gmp-guideline/eu-gmp-annex-2-manufacture-of-biological-medicinal-products-for-human-use.
2 Langer ES, et al. 19th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production. BioPlan Associates: Rockville, MD, 2021, https://www.bioplanassociates.com/19th.
3 Process Intensification: Key Considerations and Expert Insights. Sartorius, 2021: https://www.sartorius.com/en/723616-723616.
4 Tindal S, et al. Intensifying Upstream Processing: Implications for Media Management. Sartorius, 2021; https://www.sartorius.com/en/791518-791518.
Basak Temel ([email protected]) is a manager of process consulting, and Jacob Slivka ([email protected]) is the head of integrated solutions sales at Sartorius. Katy McLaughlin ([email protected]) is a scientific content writer at Sartorius.
You May Also Like





