SARS-CoV-2 Hyper-Immunoglobulin: Purification and Characterization from Human Convalescent Plasma
 The novel severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) emerged as a major pandemic coronavirus disease in 2019 (COVID-19) and since then has killed many people and paralyzed the global economy (1, 2). With specific antiviral therapeutic agents or antibodies yet to be approved, other antivirals and novel vaccine strategies have been essential to containing the virus and disease transmission.
The novel severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) emerged as a major pandemic coronavirus disease in 2019 (COVID-19) and since then has killed many people and paralyzed the global economy (1, 2). With specific antiviral therapeutic agents or antibodies yet to be approved, other antivirals and novel vaccine strategies have been essential to containing the virus and disease transmission.
Passive antibody therapy can be used to limit the scope of epidemics by providing patients with antibodies that recognize epitopic regions of virus particles to reduce their replication and consequently lessen the severity of viral disease. Antibodies for passive immunotherapy can be manufactured through recombinant DNA technology or isolated from the blood of recovered patients. The conventional strategy using convalescent plasma (CP) isolated from treated patients has improved survival rates in several viral infection events involving Machupo virus (Bolivian hemorrhagic fever) (3), Junín virus (Argentinian hemorrhagic fever) (4), Lassa fever (5), and Ebola virus (6). CP therapy also was used to treat patients with SARS-CoV-1 infection (known also simply as SARS) and Middle East respiratory syndrome (MERS), with promising results (7, 8). Due to increased demand for remdesivir antiviral therapy and limited resources in many regions, CP was considered for patients stricken with moderate and severe COVID-19 (9).
CP therapy brings with it some associated disadvantages. The most common adverse reactions are transfusion-related chills and fever, anaphylactic reactions, acute lung injury, circulatory overload, and hemolysis (10). And drug developers should not overlook the risk of transfusion-transmitted infections such as human immunodeficiency virus (HIV), hepatitis B and C viruses (HBV, HCB), and syphilis (10).
Early use of CP for COVID-19 did not yield desirable outcomes. Clinical trial results demonstrated that CP use improved resolution of both fatigue and shortness of breath, reduced supplemental oxygen requirements in the first week of treatment, and increased negative conversion of viral RNA. But those effects did not reduce 28-day mortality or lessen the progression to severe disease in moderate COVID-19 patients (11), possibly because titers of neutralizing antibodies (NAbs) were low with conventional CP.
Monoclonal antibody (MAb) cocktails exhibit more potent antivirus activity that could increase the effectiveness of treatment and prevent viruses from escaping antibody neutralization (12). The most effective treatment options against SARS-CoV-2 are based on the use of broad-spectrum antiviral drugs or the use of specific therapeutic molecules that directly interrupt either certain stages of the viral lifecycle or the receptor proteins located on the surface of host cells. Prevention of virus binding, attachment, and entry into cells can be achieved using peptide fusion inhibitors, SARS-CoV-2 NAbs, MAbs against angiotensin-converting enzyme 2 (ACE2), and protease inhibitors. Developing specific preventive and therapeutic intervention strategies — including vaccines, MAbs, peptides, interferon therapies, and small-molecule drugs to combat SARS-CoV-2 — will take time and depend on results of clinical testing.
Meanwhile, broad-spectrum, polyclonal, and diverse NAbs remain a promising option to minimize the chances of viral escape. Such antibodies can be purified and concentrated from pooled CP sourced from recovered patients and could be more beneficial than classical CP therapy. The resulting drug product would provide more consistent antibody levels in each lot, and units would be easy to store, distribute, and administer to patients. Companies such as Kamada in Israel have developed SARS-CoV-2 antibodies from CP and are assessing the safety, pharmacokinetics, and pharmacodynamics of plasma-derived hyper-immunoglobulin (hyper-IgG) in clinical trials.
Here, we report on our own development and characterization of a safe and efficient preparation of SARS-CoV-2 hyper-IgG. Immunoglobulin purification typically involves time-consuming fractionation of plasma and chromatography steps. Our process bypasses plasma fractionation by precipitating out contaminating proteins using octanoic-acid treatment. We use a protein A affinity chromatography column to capture and purify immunoglobulins for maximum product recovery and impurity removal in a single step. Viral safety is ensured by two additional orthogonal steps for enveloped and nonenveloped viruses.
During product development, we screened many analytical techniques for measuring total NAbs against SARS-CoV-2. Enzyme-linked immunosorbent assays (ELISAs) and lateral-flow immunodiffusion assays can detect total binding antibodies but cannot differentiate between those and NAbs against SARS-CoV-2. We identified a more consistent, simple, fast, effective, specific, and sensitive means for quantifying NAbs against S1 and S2 antigens of the virus using an automated and contained serological chemiluminescence immunoassay (CLIA).
We used a surrogate virus-neutralization assay to assess the ability of our product to inhibit entry of SARS-CoV-2 viruses into host cells by blocking the interaction between human ACE2 with NAbs against the receptor-binding domain (RBD) of the virus. To ensure product efficacy, we also performed serum-neutralization and plaque assays using live virus. Other product quality attributes (PQAs) were evaluated by tests similar to those used for normal human immunoglobulins according to the European Pharmacopoeia.
Selection of Plasma Donors |
|---|
Plasma was collected from government-approved blood banks with the following criteria applied to screening donors (1): In addition, donor eligibility criteria for whole-blood donation were followed in accordance with the 1940 Drugs and Cosmetics Act of India and 1945 rules therein. Viral safety of plasma was ensured by testing individual units for antibodies against viruses and for viral nucleic acids. Reference1 Agarwal A, et al. Convalescent Plasma in the Management of Moderate COVID-19 in Adults in India: Open Label Phase II Multicentre Randomised Controlled Trial (PLACID Trial). BMJ October 2020: 371; https://doi.org/10.1136/bmj.m3939. |
Materials and Methods
Materials: We obtained source/recovered CP in polyvinyl bags from Indian blood banks approved by the Drug Controller General of India (DCGI) and registered with the National AIDS Control Organization under the Government of India. The “Selection” box provides more information.
To test for HIV1, HIV2, and HCV, we used antibody test kits from Bio-Rad Laboratories; for HBV, we used a kit from Diagnostic BioProbes (Dia.Pro). Plasma also was tested for HBV, HCV, and HIV1 using standard nucleic-acid test kits from Roche according to US Food and Drug Administration (FDA) protocols.
Columns, resins, and chromatography skids used in the purification process came from Cytiva. Planova 20N virus filters came from Asahi Kasei Medical. For concentration and buffer exchange, we used a tangential-flow filtration (TFF) system and 50-kDa molecular weight cut-off (MWCO) membrane from EMD Millipore. For IgG measurements and differentiation of subclasses, we used an Atellica NEPH 630 nephelometer system from Siemens Healthineers India. For total protein estimation, we used Kjeldahl equipment from Gerhardt Analytical Systems.
The minisubmarine electrophoresis unit that we used for immunoelectrophoresis came from B Genie, and our semi-micro-osmometer came from Knauer. For protein composition estimation, we used a Hydrogel K20 applicator and accessory kit from Sebia.
We obtained anti-A and anti-B hemagglutinins and anti-D antibodies from Tulip Diagnostics to use as controls. Dia.Pro provided a kit for measurement of antibodies to HBV surface antigen (HBsAg). For measurement of anticomplementary activity, we used hemolysin from Sigma-Aldrich and complement from Cedarlane.
To detect prekallikrein activator (PKA), we used a kit from Pathway Diagnostics. We also used an IgA estimation kit from Cygnus Technologies. A high-performance size-exclusion liquid chromatography (SEC-HPLC) column came from Tosoh Biosciences. Chemicals for preparing mobile-phase phosphate-buffered saline (PBS) came from EMD Millipore. NAbs were detected by a Liaison XL analyzer from Diasorin. And we used a SARS-CoV-2 surrogate virus-neutralization test kit from GenScript USA.
Plasma Testing: Every unit of plasma sourced for purification of SARS-CoV-2 hyper-IgG was tested for the presence of antibodies against HIV1, HIV2, HBV, and HCV. Units showing no antibodies against those viruses were tested further for viral nucleic acids. All units that tested negative were pooled and used for purification of SARS-CoV-2 hyper-IgG. These tests were performed in addition to certifications provided by the different blood banks.
Purification Process Development: Our IgG purification process includes two virus-clearance methods. The development process prioritized viral safety, maximum purity, and maximum recovery. We used octanoic acid as a virus-inactivation agent that also provided partial purification of immunoglobulins upstream of protein A affinity chromatography.
Briefly, CP was thawed, pooled, and reconditioned to the requirements of octanoic acid and impurity precipitation (13). Reconditioned plasma was diluted with equal volumes of buffer, and octanoic acid was added slowly to a final concentration of 2% v/v. The resulting solution was incubated at ambient temperature for an hour with continuous stirring. Precipitates thus formed were removed by centrifugation, and supernatant was filtered through a 2-µm depth filter.
Clarified supernatant was loaded onto an equilibrated protein A affinity column to maximize IgG recovery while allowing contaminating proteins to flow through. IgG elution was achieved by lowering column pH with a glycine buffer. We further purified the affinity column eluent on an anion-exchange (AEX) column, allowing IgG to flow through the column. Bound impurities were eluted by raising the solution conductivity. AEX flow-through was filtered through a virus-retentive Planova 20N filter to remove viruses, if any were present.
We concentrated the filtered solution to ~10% IgG and buffer-exchanged it against 0.3 M glycine, then filtered the final concentrated IgG solution through a 0.2-µm filter into vials.
The resulting product was characterized extensively using methods prescribed in both the Indian and European pharmacopoeias for testing IgG preparations to be used intravenously. We also assessed SARS-CoV-2 NAbs using orthogonal tools to establish the hyperimmune nature of the product.
Determination of SARS-CoV-2 NAbs: For quantitative analysis to detect SARS-CoV-2 neutralizing IgG antibodies against subunits S1 and S2 of the virus spike protein, we used a Liaison XL analyzer according to the manufacturer’s instructions. This diagnostic test is approved by both the US FDA and the European Medicines Agency (EMA).
Briefly, recombinant S1 and S2 antigens are coated on magnetic particles so that SARS-CoV-2 IgG antibodies present in calibrators, samples, or controls bind to them. Mouse MAbs against human IgG linked to an isoluminol derivative react with the IgGs already bound to those antigens. Starter reagents are added to induce a flash chemiluminescence reaction. The light signal is measured by a photomultiplier as relative light units (RLU), indicating the presence of IgGs against SARS-CoV-2. Results are reported in arbitrary units per milliliter, which indicates the reaction intensity to measure the neutralizing levels.
SARS-CoV-2 Surrogate Virus Neutralization Test: The GenScript SARS-CoV-2 surrogate virus neutralization test (sVNT) kit can detect circulating NAbs against SARS-CoV-2 that block interactions between the RBD of the viral spike glycoprotein with the ACE2 cell surface receptor. This assay detects antibodies that neutralize those interactions. We performed the assay according to the manufacturer’s instructions using a UK National Institute for Biological Standards and Control’s (NIBSC’s) 20/130 standard for anti-SARS-CoV-2 antibodies as a positive control (to demonstrate system suitability) in addition to positive and negative controls supplied in the kit. Absorbance of samples depends inversely on the titer of NAbs present. We calculated our results based on optical density (OD) values for the samples — with negative controls based on a formula included in the kit instructions — and then interpreted our data in terms of percentage of virus inhibition.
Serum Microneutralization and Plaque Reduction Neutralization Assay: For product efficacy determination, purified preparations along with positive and negative controls were analyzed by both serum microneutralization and plaque assays using live virus. This work was performed at the Translational Health Science and Technology Institute (THSTI), an autonomous institute under the department of biotechnology in India’s Ministry of Science and Technology.
The serum-neutralization titer (SNT) assay determines neutralizing antibody titers against SARS-CoV-2 in a sample by an in vitro serum-neutralization assay using Vero/Vero-E6 cells in 96-well microplates. Inactivated samples were diluted serially in Dulbecco’s Modified Eagle’s Medium (DMEM) and mixed with diluted virus stock (1 × 102 TCID50 per 50 µL). The mix of virus and serum dilutions was added to Vero-E6 cells in respective wells of the plate and incubated at 37 °C with 5% CO2 for an hour. Cells in each well were washed, then analysts added Eagle’s minimum essential medium (MEM) with 2% fetal calf serum (FCS) to each one. The plate was incubated at 37 °C with 5% CO2 for 72–96 hours before cells were examined microscopically for virus-induced cytopathic effect (CPE).
The plaque-reduction neutralization test (PRNT90) determines relative concentrations of virus-specific NAbs in a serum sample by measuring the titer at which a serum sample produces 90% reduction in plaques. This assay used Vero-E6 cells in 24-well plates. Heat-inactivated samples were diluted serially in MEM with 2% FCS medium and mixed (1:1) with 150 µL of diluted virus stock containing 20–30 plaque-forming units (PFUs) per 150-µL, then incubated at 37 °C with 5% CO2 for an hour. Afterward, 150 μL of the virus–sample-dilution mix was added to the wells of a 24-well culture plate with a Vero-E6 cell monolayer. That plate was incubated at 37 °C with 5% CO2 for an hour before the cells in each well were washed, then analysts added MEM with 2% FCS supplemented by carboxymethyl cellulose (CMC) to each well. After 72 hours incubation at 37 °C with 5% CO2, the cells were fixed and inactivated using 3.7% formaldehyde. The plate was incubated at room temperature for 30 minutes, then the cell monolayer was stained using crystal (Gentian) violet stain for an hour. Finally, plate wells were washed with water before plaques were counted on a light box.
Manual plaque counts were combined with dilution factors to calculate the number of PFU per sample unit volume. That result represents the number of infective particles within a sample and is based on the assumption that each plaque formed represents one infective virus particle. Comparing the serum concentration needed to reduce the number of plaques by 90% from concentrations obtained with serum-free virus gives a measure of how much antibody is present and/or how effective it is, denoted as the PRNT90 value.
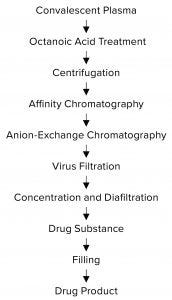
Figure 1: Purification process for SARS CoV-2 hyper-immunoglobulin
Antibodies and Proteins: To measure total IgG, we followed manufacturers’ instructions using an Atellica NEPH 630 nephelometer (Siemens Healthineers) with a kit supplied by Siemens. The same instrument was used to quantify IgG 1–4 subclasses present. They form insoluble immune complexes that cause light scattering, measurement of which enumerates their concentrations. A log–log regression through six points gives a reference curve on which unknown samples can be quantified. We used immunoelectrophoresis on a minisubmarine electrophoresis unit to identify IgG with rabbit antisera against total human and animal proteins.
Total protein was determined by the Kjeldahl method, using an internal reference standard for system suitability and a semiautomated instrument from Gerhardt Analytical Systems. We used zone electrophoresis per manufacturer’s instructions to determine protein composition using a Sebia Hydragel K20 accessory kit.
We measured anti-A and anti-B hemagglutinins by the indirect Coombs method, using controls from Tulip Diagnostics for system suitability. We applied a test procedure from the Indian and European pharmacopoeia to determine the presence or absence of anti-D antibodies, with a system suitability standard from NIBSC (05/242) during the assay.
Antibodies to the HBsAg were measured following the protocol established by Dia.Pro Diagnostics. Anticomplementary activity was determined by incubating 10 mg of IgG with a defined amount of guinea-pig complement (20 CH50) from Cedarlane and titrating the remaining complement to provide results as a percentage consumption of the control.
We tested for PKA using a PW 2302 chromogenic substrate from Pathway Diagnostics with respect to the rate at which the p-nitroaniline was released (measured photometrically at 405 nm). And we tested for the presence of other plasma proteins (e.g., IgA) using an ELISA from Cygnus Technologies based on the manufacturer’s instructions.
We applied chromatographic separation to determine the molecular-size distribution of proteins, running a 30-cm × 7.8-mm ID Tosoh column with 5-µm particles of 250 Å porosity using phosphate-buffered saline (PBS) at pH 7.0. Samples were normalized to 10 mg/mL in the mobile phase, with 100 µL of sample injected at 0.5 mL/min and absorbance measured at 280 nm.
Osmolality: Sample osmolality was measured with a semimicro-osmometer from Knauer based on manufacturer’s instructions. We used standards supplied by Knauer for quality control during this procedure.
Viral Testing and Product Safety: We ensured biological safety of our product by nonreactivity in ELISA for HBsAg, HIV-1, and HIV-2, and HCV antibodies and by negative results for HIV-1, HIV-2, HCV, and HBV in nucleic-acid testing.
Safety of the drug was evaluated further with pyrogen testing in rabbits and abnormal toxicity analysis using guinea pigs and mice. These tests were conducted at a government-approved third-party laboratory.
 Results
Results
Purification: Figure 1 summarizes the purification process. SARS-CoV-2 hyper-IgG is purified by a combination of antibody adsorption and desorption on different chromatographic resins. We use a protein A affinity column to maximize IgG recovery from CP. AEX chromatography provides further polishing of the affinity-purified IgG. This purification process also includes two specific orthogonal steps for virus inactivation and removal: octanoic-acid treatment and nanofiltration.
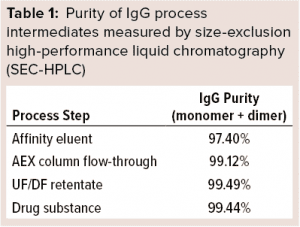
SARS-CoV-2 hyper-IgG recoveries were ~60% of the initial IgG quantities in our source plasma. Octanoic-acid treatment enabled removal of major impurities before the affinity step, allowing for minimum nonspecific interaction of remaining impurities with the column. IgG recoveries from the chromatography steps were >85%. Table 1 delineates the purity of each process intermediate.
SARS-CoV-2 NAbs: We needed effective, sensitive, and specific means for identification and laboratory confirmation of SARS-CoV-2 NAbs in our CP purified preparation. Many commercial antibody assays are available, but none yet had been fully validated. The US FDA granted emergency use authorization (EUA) for multiple tests while stressing the need for further validation (14, 15). To address our need for reliable antibody-based quantification assays, we focused on the RBD of the coronavirus spike protein because that region is conserved poorly between different virus species and is known to be a major target of human antibodies (16).
We identified anti-S1 and anti-S2 specific NAbs with an indirect CLIA from Diasorin that is validated for quantification of NAbs against SARS-CoV-2 S1 and S2 antigens. According to the manufacturer, the test discriminates negative (<12 AU/mL) from equivocal (12.0–15.0 AU/mL) and positive (>15.0 AU/mL) results. It is a high-throughput (170 tests/hour), automated method that requires no manual intervention.
We qualified our assay for specificity, repeatability, and intermediate precision. It proved to be specific in detecting SARS-CoV-2 NAbs without matrix interference from formulation buffer or nonspecific samples. Precision was evaluated by determining repeatability (3.6% SD) and intermediate precision (6.0% SD).
CP already is used to treat COVID-19 patients, but the NAb content of plasma varies from patient to patient. That makes it necessary to screen CP for NAb titers and ensure fixed amounts of antibodies in a hyperimmune product. We tested many units of CP, finding the NAb contents to be ~60 AU/mL. SARS-CoV-2 antibody levels in our final preparation ranged 245–455 AU/mL (Table 2). These results proved our purified hyper-IgG preparation to be rich in NAbs against the coronavirus spike proteins with at least 5× more NAbs than are recommended for CP (13 AU/mL by CLIA) (17).
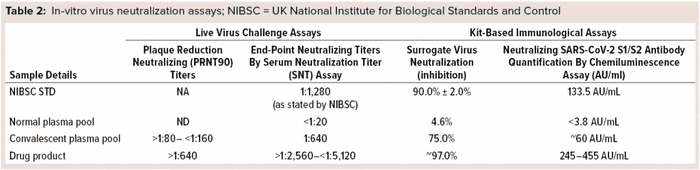 Antibody-Mediated Neutralization of Virus-Receptor Interaction: Another method of assessing the ability of this antibody preparation to neutralize the virus is the sVNT. In COVID-19 infections, human ACE2 is the main functional receptor for viral entry (16). The RBD of the SARS-CoV-2 S protein interacts strongly with human ACE2 receptors on host cells of the deep lung. Secreted NAbs from the natural immune response provide protection against future infections from viruses by remaining in a patient’s circulatory system for months to years after infection, ready to bind quickly and strongly to new pathogens and block cellular infiltration and replication. To detect those antibodies, the SARS-CoV-2 sVNT blocking ELISA mimics that virus neutralization process. Protein–protein interactions between horseradish-peroxidase–RBD and human ACE2 can be blocked by NAbs against SARS-CoV-2 RBD. Our results confirmed that, with both CP and final purified preparations showing 75% inhibition of surrogate virus in CP, and final purified preparations providing ~97% inhibition of surrogate virus (Table 2).
Antibody-Mediated Neutralization of Virus-Receptor Interaction: Another method of assessing the ability of this antibody preparation to neutralize the virus is the sVNT. In COVID-19 infections, human ACE2 is the main functional receptor for viral entry (16). The RBD of the SARS-CoV-2 S protein interacts strongly with human ACE2 receptors on host cells of the deep lung. Secreted NAbs from the natural immune response provide protection against future infections from viruses by remaining in a patient’s circulatory system for months to years after infection, ready to bind quickly and strongly to new pathogens and block cellular infiltration and replication. To detect those antibodies, the SARS-CoV-2 sVNT blocking ELISA mimics that virus neutralization process. Protein–protein interactions between horseradish-peroxidase–RBD and human ACE2 can be blocked by NAbs against SARS-CoV-2 RBD. Our results confirmed that, with both CP and final purified preparations showing 75% inhibition of surrogate virus in CP, and final purified preparations providing ~97% inhibition of surrogate virus (Table 2).
Conventional VNTs also detect NAbs but require live viruses and/or cells used in a biosafety level 3 (BSL-3) facility, making testing time consuming and laborious. The sVNT is performed without live virus or cells and can be completed easily in a standard QA/QC laboratory within about two hours.
Serum-Microneutralization and Plaque-Reduction–Neutralization Assays: To prove product efficacy, we tested our final purified hyper-IgG using live virus culture. Samples were evaluated for the presence of NAbs against SARS-CoV-2 virus using serum-microneutralization and plaque-reduction–neutralization assays. NAbs were quantified in different amounts of both CP and final purified hyper-IgG preparation (Table 2). NAb titers observed in the serum microneutralization assay for final purified samples were >1:2,560 to <1:5,120 (1:640 for CP samples). PRNT90 values in final purified preparation were >1:640 (>1:80 to <1:160 in CP samples).
Those results indicate that the hyper-IgG preparation is highly effective in neutralizing live virus. Furthermore, total protein content of the product was ~10% (100 mg/mL), which shows its resemblance to existing 10% total protein in other IgG preparations for intravenous use (Table 3).
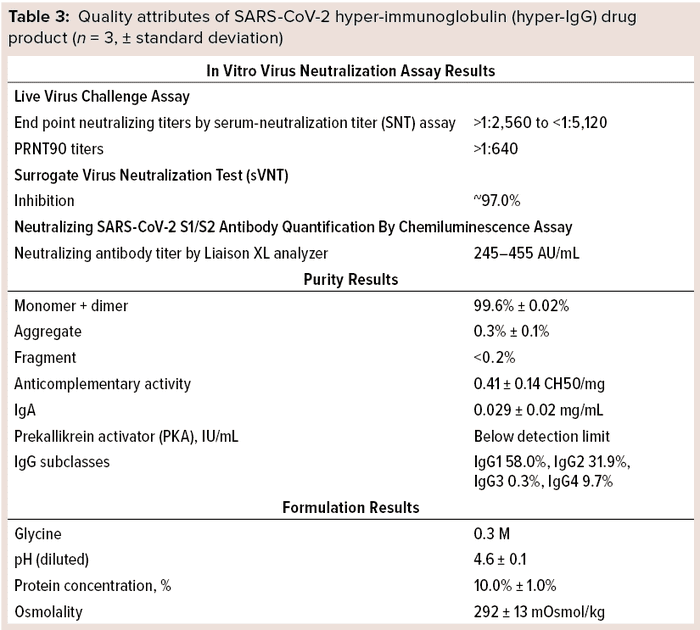 Quality Testing: We evaluated other quality attributes of our final purified preparation as summarized in Table 3. In immunoelectrophoretic identification, a precipitin arc was observed with use of antihuman rabbit sera. SEC-HPLC revealed that the final preparation was extremely pure, with >99% desirable forms (monomers and dimers) of IgG. Further, protein composition by zone electrophoresis showed the principal band to be >96% pure. Osmolality of the final product was >240 mOsmol/kg.
Quality Testing: We evaluated other quality attributes of our final purified preparation as summarized in Table 3. In immunoelectrophoretic identification, a precipitin arc was observed with use of antihuman rabbit sera. SEC-HPLC revealed that the final preparation was extremely pure, with >99% desirable forms (monomers and dimers) of IgG. Further, protein composition by zone electrophoresis showed the principal band to be >96% pure. Osmolality of the final product was >240 mOsmol/kg.
Anti-A and anti-B hemagglutinin testing by the indirect Coombs method showed all dilutions tested to be free from agglutination. Additionally, the final purified preparations were free of anti-D antibodies, showing no agglutination with O+ or O– red blood cells sensitized by papain. Over 0.5 IU/g of antibodies detected were those against HBsAg.
Furthermore, we estimated the anticomplementary activity in our product to be ≤50% (1 CH50/mg of immunoglobulin). No PKA could be detected in most samples. ELISA-determined IgA content was much lower (~0.029 mg/mL) in all preparations of final purified product, and IgG subclasses were measured by nephelometry to be 58.0% IgG1, 31.9% IgG2, 0.3% IgG3, and 9.7% IgG4.
We tested our final product for overall safety and viral pathogens. Final purified preparations tested negative and nonreactive, ensuring viral safety of the product. In addition, we tested final purified SARS-CoV-2 hyper-IgG for pyrogens using rabbits and for abnormal toxicity using mice and guinea pigs. Total temperature-rise responses in three rabbits were ≤1.4 °C; temperature rise response of individual rabbits was <0.6 °C. No guinea pigs or mice died or showed signs of ill health over seven days following injection of samples.
Discussion
COVID-19 has emerged as a major global healthcare challenge. Although confirmed cases have accumulated rapidly over the past year, our understanding of the clinical spectrum and pathophysiological changes of this infection remains limited. No definitive treatment has been identified, making clinical management extremely difficult. Purified hyper-IgG therapy is an advanced version of CP treatment with many advantages over conventional plasma therapy: e.g., lower administration volumes, consistent dosage amounts, negligible risk of virus transmission, and no requirement of blood-group subtyping. In addition, removing IgM during purification of hyper-IgG products protects patients from adverse reactions such as anti-A and anti-B intravascular activity (18).
Similarly, removing IgA provides a therapeutic advantage for IgA-deficient patients who may have been treated with blood products and developed antibodies to IgA (19).
Our purified SARS-CoV-2 hyper-IgG preparation is rich in NAbs against COVID-19. They can block progression of the disease cascade early in infections and eventually should improve outcomes. The product exhibits high virus-neutralization ability as demonstrated using assays with live virus. That clearly qualifies it as an efficient candidate for NAb therapy. Hence, the efficacy and quality parameters (Tables 2 and 3) give us confidence to offer this product as an option for treating COVID-19 patients. The SARS-CoV-2 hyper-IgG drug product also has been developed successfully as a passive vaccine that is safe from both potential viral pathogens and free of toxicity.
Broad efficacy of this product comes from a large source pool of CP from patients in different regions who may have encountered different strains of the COVID-19 virus. The pooled plasma includes a large repertoire of antibodies from different strains of virus to provide broad-spectrum protection against a wide range of SARS-CoV-2 strains that are circulating in the population.
Hyper-IgG also precludes the need for patients to search for CP donors. Because the time of treatment initiation after infection plays a key role in the patient recoveries, searching for CP from matching donors can delay treatment and aggravate the conditions of some patients. With SARS-CoV-2 hyper-IgG therapy, treatment can be made available immediately, which should aid in faster recoveries. This product also can be stored at 2–8 °C for longer periods than CP and can be transported easily.
Interim results from ongoing clinical trials (CTRI/2020/09/027903) for this newly developed SARS-CoV-2 hyper-IgG product demonstrate its efficiency in limiting viral infection if administered early from the day of disease onset (data not published). This preparation will be explored for prophylactic use as a passive vaccine, which could reduce risk for members of the medical community who work directly or indirectly with patients and/or vulnerable populations.
References
1 Huang C, et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. The Lancet 395(10223) 2020: 497–506; https://doi.org/10.1016/S0140-6736(20)30183-5.
2 Ren LL, et al. Identification of a Novel Coronavirus Causing Severe Pneumonia in Human: A Descriptive Study. Chinese Med. J. 133(9) 2020: 1015–1024; https://doi.org/10.1097/CM9.0000000000000722.
3 Stinebaugh BJ, et al. Bolivian Hemorrhagic Fever: A Report of Four Cases. Am. J. Med. 40(2) 1966: 217–230; https://doi.org/10.1016/0002-9343(66)90103-3.
4 Ruggiero HA, et al. Treatment of Argentine Hemorrhagic Fever with Convalescent’s Plasma 4433 Cases [in French]. Presse Med. 15(45) 1986: 2239–2242; https://pubmed.ncbi.nlm.nih.gov/2949253.
5 Frame JD, et al. The Use of Lassa Fever Convalescent Plasma in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 78(3) 1984: 319–324; https://doi.org/10.1016/0035-9203(84)90107-x.
6 Mupapa K, et al. Treatment of Ebola Hemorrhagic Fever with Blood Transfusions from Convalescent Patients. J. Infect. Dis. 179(S1) 1999: 18–23; https://doi.org/10.1086/514298.
7 Mair-Jenkins J, et al. The Effectiveness of Convalescent Plasma and Hyperimmune Immunoglobulin for the Treatment of Severe Acute Respiratory Infections of Viral Etiology: A Systematic Review and Exploratory Meta-Analysis. J. Infect. Dis. 211(1) 2015: 80–90; https://doi.org/10.1093/infdis/jiu396.
8 Marano G, et al. Convalescent Plasma: New Evidence for an Old Therapeutic Tool. Blood Transfus. 14(2) 2016: 152–157; https://dx.doi.org/10.2450%2F2015.0131-15.
9 Maor Y, et al. Compassionate Use of Convalescent Plasma for Treatment of Moderate and Severe Pneumonia in COVID-19 Patients and Association with IgG Antibody Levels in Donated Plasma. EClin. Med. 26, September 2020: 100525; https://doi.org/10.1016/j.eclinm.2020.100525.
10 Zhao Q, He Y. Challenges of Convalescent Plasma Therapy on COVID-19. J. Clin. Virol. 127, June 2020: 104358; https://dx.doi.org/10.1016%2Fj.jcv.2020.104358.
11 Nagoba B, et al. Positive Aspects, Negative Aspects and Limitations of Plasma Therapy with Special Reference to COVID-19. J. Infect. Pub. Health 13(12) 2020: 1818–1822; https://dx.doi.org/10.1016%2Fj.jiph.2020.08.011.
12 Shanmugaraj B, et al. Perspectives on Monoclonal Antibody Therapy As Potential Therapeutic Intervention for Coronavirus Disease-19 (COVID-19). Asian Pac. J. Allergy Immunol. 38(1) 2020: 10–18; https://doi.org/10.12932/ap-200220-0773.
13 Iler HD, Rudnick D, Kloft M. Inactivation of Lipid Enveloped Viruses By Octanoic Acid Treatment of Immunoglobulin Solution. Biologicals 30, 2002: 135–142; https://doi.org/10.1006/biol.2002.0332.
14 Okba NMA, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Specific Antibody Responses in Coronavirus Disease 2019 Patients. Emerg. Infect. Dis. 26, 26(7) 2020: 1478–1488; https://dx.doi.org/10.3201/eid2607.200841.
15 Dohla M, et al. Rapid Point of Care Testing for SARS-CoV-2 in a Community Screening Setting Shows Low Sensitivity. Pub. Health 182, May 2020: 170–172; https://doi.org/10.1016/j.puhe.2020.04.009.
16 Du L, et al. The Spike Protein of SARS-CoV: A Target for Vaccine and Therapeutic Development. Nat. Rev. Microbiol. 7, 2009: 226–236; https://doi.org/10.1038/nrmicro2090.
17 Evidence Based Advisory to Address Inappropriate Use of Convalescent Plasma in COVID-19 Patients. Indian Council of Medical Research: New Delhi, India, 17 November 2020; https://www.icmr.gov.in/pdf/covid/techdoc/ICMR_ADVISORY_Convalescent_plasma_17112020_v1.pdf.
18 Flegel WA. Pathogenesis and Mechanisms of Antibody-Mediated Hemolysis. Transfusion 55(S2) 2015: S47–S58; https://doi.org/10.1111/trf.13147.
19 Pineda AA, Taswell HF. Transfusion Reactions Associated with Anti-IgA Antibodies: Report of Four Cases and Review of the Literature. Transfusion 15(1) 1975: 10–15; https://doi.org/10.1046/j.1537-2995.1975.15175103503.x.
Further Reading
Data File 28-9870-62 AA. Affinity Chromatography: MabSelect SuRe™ LX. GE Healthcare Bio-Sciences: Uppsala, Sweden, February 2011; https://www.cytivalifesciences.co.jp/catalog/pdf/mabselect_sure_lx.pdf.
Korneyeva M, et al. Enveloped Virus Inactivation by Caprylate: A Robust Alternative to Solvent-Detergent Treatment in Plasma Derived Intermediates. Biologicals 30, 2002: 153–162; https://doi.org/10.1006/biol.2002.0334.
Liu HF, et al. Recovery and Purification Process Development for Monoclonal Antibody Production. mAbs 2(5) 2010: 480–499; https://dx.doi.org/10.4161%2Fmabs.
2.5.12645.
Parkkinen J, et al. A Modified Caprylic Acid Method for Manufacturing Immunoglobulin G from Human Plasma with High Yield and Efficient Virus Clearance. Vox Sanguinis 90, 2006: 97–104; https://doi.org/10.1111/j.1423-0410.2005.00731.x.
Sachin Verma is a principal scientist; Sheetal Dolia is assistant general manager of research and development; Amit Pawar is deputy general manager of the manufacturing department; and corresponding author Suma Ray, PhD, is senior vice president of the plasma fractionation unit at Intas Pharmaceuticals, Ltd. Plot 496/1/A&B, Sarkhej Bavla Highway, Village Matoda, Taluka Sanand, Ahmedabad, Gujurat, India 382213; 91-02717-662135; [email protected]; https://www.intaspharma.com.
You May Also Like





