Exploring Academic Models for Biomanufacturing Education

Students in the Master of Microbial Biotechnology (MMB) program at NCSU.
Undergraduate and/or graduate programs in physical and life sciences can provide a solid background for science and engineering students who are interested in careers in biotechnology research and development. Yet many such programs have not adequately prepared students for careers within and related to the biopharmaceutical industry.
In today’s globally competitive job market, developing a workforce pipeline for the bioprocess industry requires academic programs that equip students with knowledge, skills, and theory surrounding the equipment, methodologies, processes, and regulatory requirements specific to it. Informed graduates are better able to manage their own career expectations, understand job requirements, and get their “foot in the door.”
Universities, community colleges, and even high schools have recognized the need for a college-educated and highly skilled workforce and have worked to develop industry-focused academic models. The focus herein is to identify and highlight some of the best practices that have ensured program success and industry relevance. Among them are the following:
Instructing students using industry-relevant equipment to provide ample hands-on experience
Instilling foundational business, leadership, and management skills
Attracting, engaging, and training nontraditional audiences for careers in biomanufacturing
Making competitive academic programs more cost effective for students
Exposing students to the global workplace through immersive and internationally based internships
Ensuring that teachers are equipped with bioprocessing knowledge and skills and that laboratories are furnished with the appropriate equipment.
For this special issue, BTEC invited contributors from a number of academic and training institutions (listed here in order of their sections, below):
Rick Lawless and Patty Brown (BTEC and NCSU)
Mike Fino (Miracosta College, CA)
Jim DeKloe (Solano College, CA)
Paul Hamilton and Jason Cramer (Master of Microbial Biotechnology program at NCSU)
Jenny Ligon (National Center for Therapeutics Manufacturing at Texas A&M University)
Susanna Leong and Lim Kok Hwa (Singapore Institute of Technology).
Hands-On Success
Patty Brown and Rick Lawless
Consider this for a minute: What do an individual learning to drive, a professional musician, a medical student, and an Olympic athlete have in common? The mental and physical capabilities to perform as required? (Of course.) Determination and persistence? (Certainly.) Patience? (Let’s hope so.)
Each individual also needs to practice regularly. Each activity — whether it’s learning to drive, playing a musical instrument, carrying out a medical procedure, or performing as a competitive athlete — requires a certain set of skills, and those skills must be learned, developed, and maintained over time. That requires practice.
The dictionary definition of skill refers to the ability to use knowledge effectively and readily in execution or performance; dexterity or coordination especially in the execution of learned physical tasks; and a learned power of doing something competently: a developed aptitude or ability (1). Hands-on practice is integral to skill development. It’s exceedingly unlikely that a new driver could operate a car with a manual transmission after simply reading about how the clutch and gearshift work. (At least, most drivers wouldn’t want to stop on a hill behind that vehicle at a red light.) Similarly, a medical student isn’t adequately prepared to perform a procedure on a patient after having attended only a lecture or seen a demonstration. Instead, both the driver and medical student need to learn the tasks involved and practice repeatedly while supervised, getting feedback along the way to correct errors and improve their performances. People with highly developed skills, such as professional musicians or Olympic athletes, also require regular practice to maintain and enhance those skills.
Experiential or hands-on learning — guided practice, to be specific — is a key feature of almost all courses at BTEC in Raleigh, NC. The center’s mission is to provide skilled professionals for the biomanufacturing industry, and both its curriculum and facilities are designed to support that mission. The curriculum includes undergraduate and graduate programs as well as courses for industry professionals, with emphasis on hands-on instruction. Course instructors are highly qualified scientists and engineers with industry expertise, and they are aided by an experienced operations staff.
To meet industry demand for students skilled in operating equipment and processes unique to biomanufacturing, BTEC provides its hands-on training in facilities furnished with US$16 million of industry-standard equipment. An 82,500 gross-square-foot (GSF) main facility features laboratories with bench-scale and pilot-scale bioprocessing equipment. The largest laboratory area is a simulated current good manufacturing practices (CGMP) production facility with fermentation, recovery, and purification operations. A 5,000-GSF annex has been fitted out for viral processing, cell culture, and purification. Local industry experts provided advice on BTEC’s facility design and equipment needs, and an industry advisory board ensures that the center’s curriculum stays up to date and relevant.
BTEC’s classrooms provide students with the requisite information, concepts, and theories related to course topics. Then in laboratory activities, students apply those concepts and practice related skills while faculty and staff provide feedback. For example, in an upstream processing course, one laboratory activity requires students to work in small groups performing changeover operations and preparing a 30-L bioreactor for steam-in-place. Students learn to perform the operations required between batches and also learn where process failures can occur. To facilitate the latter, BTEC instructors can insert bad equipment or procedural errors into the exercise and then observe how students troubleshoot the problem and agree on a corrective action. In a chromatography laboratory that’s part of a downstream processing course, students execute a protocol for the operational qualification of a production-scale, low-pressure liquid chromatography system. By the end of that activity, students can identify components of the system and conduct some basic tests needed to ensure that valves, pumps, and sensors operate as intended.

Students in the MMB program at NCSU.
Emulating a Biomanufacturing Facility Environment
From the students’ point of view, hands-on learning in a workplace-like environment is often very engaging. In addition, students gain firsthand insights into the range and nature of careers available in the biopharmaceutical industry, which helps them determine their career interests. Hands-on learning also can help students interview for and secure an internship or position after graduation, especially when their learning experiences have provided familiarity with typical workplace practices and standard operating procedures (SOPs) and commonly used equipment.
From an employer’s point of view, safety concerns, expenses, and availability of equipment or other factors may make hands-on training in an actual work environment difficult. A company also might want to minimize such training to the extent possible. The availability of new graduates who are already familiar with industry equipment and standard practices provides a competitive advantage. Costs decrease as training needs are reduced, and employees are fully productive sooner than if they’d needed extensive training after hire. Employers may avoid having to address costly mistakes made by novice employees. In addition, companies could experience less turnover when new hires are more aware of job expectations and the career paths available in the industry. Finally, when preliminary training needs are adequately addressed, remaining resources can be devoted to developing employees’ more advanced competencies and problem-solving skills for process operations and troubleshooting.
BTEC’s model of providing handson education and training, which comprehensively approximates actual work experiences, is certainly exceptional. However, it’s clear that through hands-on, experiential learning, educational institutions can help deliver one of the most valuable elements in a manufacturer’s supply chain: a skilled workforce.
Capstone Instruction
Capstone instruction in general refers to a culminating and usually integrative experience as part of an educational program. An example of such instruction is the Bionetwork Capstone Center: a training center run by the NC community college system in leased space at BTEC. Its focus is on aseptic handling, gowning, and fill–finish. The courses are focused generally at the technician/operator level: www.ncbionetwork.org/educational-resources/elearning/videos/bionetwork-capstone-center. More information about the program referenced in this article can be found at www.ncbiotech.org/sites/default/files/pages/2012WindowontheWorkplace.pdf. |
Biomanufacturing Baccalaureate Degrees in California Community Colleges
Mike Fino and Jim DeKloe
Curricula focused on biomanufacturing at the community college level are not new. Although many traditional university programs emphasized the knowledge and skills to work in biotechnology research, community colleges began to emphasize technical skills and applied knowledge of biomanufacturing in two-year degree programs in the 1990s. Many such institutions worked with local biopharmaceutical companies to carve out a niche and develop curriculum to teach students the knowledge and skills to enter the field as technicians within biopharmaceutical manufacturing companies. In response to a growing demand for a highly skilled workforce with increasing levels of education, California is working proactively to reduce the cost of a four-year degree by offering industry-relevant bachelor degrees through community colleges.
On 28 September 2014, CA Governor Jerry Brown signed state Senate Bill (SB) 850 into law. This legislation authorized 15 of the 113 California community colleges to develop baccalaureate degree programs in single fields. Solano College (Fairfield) and MiraCosta College (north San Diego area) received approval to offer baccalaureate degrees in biomanufacturing.
Solano Community College is located in a suburb north of the San Francisco Bay Area. In response to an announcement that pioneering biotechnology company Genentech would build the largest multiuse cell culture manufacturing facility in the world in Solano County, the community college launched a two-year biotechnology degree program. Over time, the Genentech facility was joined by pharmaceutical manufacturing plants run by Alza Pharmaceuticals (now a division of Johnson and Johnson) and Chiron (later acquired by Novartis).
Similarly, MiraCosta College gained an interest in biomanufacturing back in 2000 when the antibody company IDEC announced its plans to build a major cell culture manufacturing facility within miles of that campus. Later Biogen merged with IDEC, and then Genentech purchased the BiogenIdec facility.
In response to industry needs, both colleges built biotechnology training facilities, one in a converted metal shop and the other in a converted automotive technology shop. The two programs coordinated their activities, and the faculty used its industry background to develop a robust biomanufacturing curriculum.
In these programs, students learn to grow genetically engineered bacterial, yeast, and mammalian cells in bioreactors; to induce them to synthesize a useful protein; to recover the proteins using centrifugation and tangential-flow filtration; to run chromatography systems and purify those proteins; and to use analytical techniques to prove the purity of the isolated protein. Students accomplish those tasks under simulated CGMP conditions. They work in shifts, follow SOPs, fill out batch records, and perform environmental monitoring.
In response to an upward shift in the educational profile of the local biopharmaceutical workforce, both college administrations believed that a logical next step would be to develop a bachelor degree in biomanufacturing through SB 850. Having gained approval to do so, both will begin offering upper-division courses in Fall 2017, and the first baccalaureate class will graduate in Spring 2019.
These community college degrees offer the advantage of low cost. The complete student tuition for a bachelor degree will be $10,500 for four years of study (estimated at approximately half the cost of a four-year degree). Every class of the associate degree will integrate seamlessly with the final two years of study because new courses will be stacked upon the current associate degree (with the biotechnology courses and concurrent general education requirements.)
The degree is designed in as practical a way as possible to promote career advancement: Many of the courses are aligned with certifications from professional organizations. The program integrates concepts from biology, chemistry, engineering, quality, and business. Each program incorporates general education courses that include technical writing, bioethics, project management, leadership, and additional biology. The biomanufacturing baccalaureate will prepare students for work within the biotechnology industry in the unique environment of biological production. In this program, science will thrive in partnership with quality and compliance with an upper division curriculum that recognizes the important roles the following elements:
Biomanufacturing science and technology
Process sciences (lecture/ laboratory)
Design of experiments for biomanufacturing (lecture/laboratory)
Design of biomanufacturing facilities, critical utilities, processes, and equipment (lecture)
Bioprocess monitoring and control (lecture/laboratory)
Capstone instruction in biomanufacturing technologies (seminar; see the “Capstone Instruction” Box)
Biomanufacturing quality
Supply chain and enterprise resource planning (lecture)
Advanced topics in quality assurance and regulatory affairs (lecture)
Six Sigma and lean manufacturing (lecture)
Methods in quality improvements, investigations, and audits (lecture)
Capstone seminar in biomanufacturing quality (seminar).
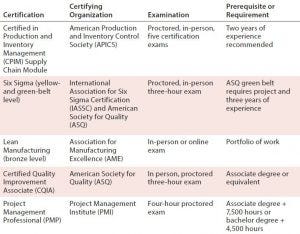
Table 1: An example of courses that align with professional certifications offered by industry organizations
Many of the new courses will be aligned with professional certifications offered by industry organizations, where possible. For example, students completing the Six Sigma/lean manufacturing course will be eligible to take the exam for a belt-level certification offered by the American Society for Quality (Table 1).
State legislation for this degree program specified that it should not be a terminal degree. In response, the degree program coordinates with both local high schools, other colleges, and professional science masters programs offered by the California State University (CSU) system to create a well-defined career pathway.
To house the program, Solano College broke ground on a new biotechnology/science building on its Vacaville campus in May 2016. Contractors anticipate that this building will be completed and ready to occupy by August 2017, coincident with the launch of the biomanufacturing baccalaureate. The building is designed with input from a biotechnology industrial advisory committee.
The building will contain four laboratory suites to teach biotechnology: a cell culture suite, a bioreactor suite, a chromatography suite, and a quality control laboratory suite. The design incorporates features of a biomanufacturing plant; that is, the building itself is a learning tool. Several million dollars of equipment have been ordered to add to current equipment used by the lower division program.
The new bachelor of science degree in biomanufacturing will provide students with another option to train for a career in biotechnology. The involvement of industry should provide students with the knowledge and practical skills required to enter and advance rapidly in the biotechnology industry.
Instilling Foundational Business, Leadership, and Management Skills
Jason M. Cramer and Paul T. Hamilton

Students in the MMB program at NCSU.
Life science companies need workers who understand the business and technical factors necessary to translate scientific discoveries into commercially viable products. Bioscience companies lean heavily on such employees and cross-functional teams to advance products and technology through their respective life cycles. As you might imagine, teams of individuals who possess strong communication and interpersonal skills along with teamwork, leadership, and problem-solving capabilities are more likely to provide support fundamental to company growth than teams lacking those skills. However, academia — which continues to serve as the primary talent pipeline for the industry — often graduates students into the workforce as highly specialized and narrowly focused individuals with limited knowledge of business practices and the professional skills valued by companies.
Professional Science Master (PSM) programs were founded to address this unmet training need and establish a pipeline of students who complete advanced training in science, technology, engineering, and mathematics (STEM) alongside development of professional skills highly valued by industry companies. In 2003, the microbiology faculty at NC State University introduced another PSM: the Master of Microbial Biotechnology (MMB) program. Like students in other PSM programs, MMB students collect science training in a variety of areas (e.g., molecular biology, biomanufacturing, statistics, and genetics), acquire key professional skills in an active learning environment, and complete a compulsory industry internship. But unlike most other PSMs, the MMB program incorporates three practicum projects into the student experience (2).
MMB students complete team-based, industry-specific projects presented during the course titled Industry Case Studies, the cornerstone of the MMB curriculum. Collaborations with local bioscience companies — including Novozymes, Biogen, Becton Dickinson, and Syngenta — establish the project work completed by MMB students. They also receive guided training to develop key professional skills throughout each of the three semesters of enrollment. Partnering companies and student team make-ups are varied each semester to increase exposure to different companies, industry sectors, and functional areas (e.g., market analysis, technology evaluation, strategic planning, and process and product development). Moreover, changing team rosters each semester fosters versatility regarding teamwork and development of interpersonal relationships. As a result of that process, MMB students complete three industry-specific projects that can be leveraged as valuable work experience while actively enrolled in the program.
For most of each 15-week semester, students work in teams to complete projects developed by partnering companies in the Research Triangle Park, NC, area. Students typically complete project work outside of class because guest presentations, professional skill development modules, and discussion of bioscience topics dominate instructional time. Formatting the course in this way establishes ample time for professional skill practice and instruction pertaining to business concepts highly relevant to bioscience companies, including those for marketing, regulatory affairs, and intellectual property.
Information we collected from life science professionals in the Raleigh–Durham region indicated that team collaboration sits atop the list of key “soft” skills necessary for employment and career advancement. Adaptability follows next, with proficiencies in spoken communication, critical analysis, written communication, building positive interpersonal relationships, time management, project management, and leadership also identified respectively. Data from local industry professionals also show that those “soft” skills are valued equally with technical skills in importance for job selection and career advancement. Thus, students who effectively display such skills are likely to experience an easier transition into industry. We implement training and evaluate development in these areas throughout each semester of Industry Case Studies. To capture evidence of student development in these areas, we use video recordings, peer and company liaison feedback, assessment of written and other course deliverables, and instructor observations.
A recent survey of MMB alumni shows that they were asked about their practicum projects during interviews both for internships and their first postgraduation jobs. To us, this shows that companies take interest in the practicum as an indicator of potential success. Alumni also reported that practicum project work enhanced their ability to land the compulsory internship required by the MMB curriculum, to gain postgraduation employment, and further, established confidence in their ability to perform initial job duties. Indeed, most MMB alumni reported specifically that project experience eased their transition to industry.
Taken together, comparing this and other programmatic data with national employment figures supports the idea that the number and quality of practicum project experiences prompt strong career outcomes for MMB students. We strongly encourage other applied and traditional programs to consider this model as a supplementary approach that might produce similar results for their graduates.
Addressing the Needs of Nontraditional Workers: Military Veterans
Jenny Ligon
Biopharmaceutical manufacturers have long recognized the hiring potential of veterans from US Armed Forces, many of whom exhibit a high level of professional polish, technical skill, and familiarity with a structured work environment. From the regulatory mindset exhibited by Navy nuclear engineers to the mechanical aptitude and troubleshooting skills that come naturally to Air Force mechanics, veterans offer great potential as a value-adding segment of the biopharmaceutical workforce. Yet for many military veterans, a lack of scientific and technical knowledge of the biomanufacturing sector presents a barrier to such employment.
In response, the National Center for Therapeutics Manufacturing (NCTM) of Texas A&M University created a program called Military Veterans Manufacturing Vaccines (MVMV) to encourage nontraditional and transitional workers to join this field. NCTM provides technical training and continuing education for the biopharmaceutical and vaccine manufacturing industries (see the “One Soldier’s Journey” box).
One Soldier’s Journey
After serving 10 years in the reserves and active duty as a supply sergeant and chemical operations specialist, Tanya Nixon was honorably discharged in 2010. While she was in the Army, Nixon’s primary responsibilities involved chemical disposal and decontamination. Her postgraduate studies in counseling, educational administration, and psychology led her to a career in education that spanned from assistant principal to dean. Although she was quite successful at it, Nixon felt that being an educator was not her true passion in life, so she sought change. When she learned of the Military Veterans Manufacturing Vaccines (MVMV) program offered by the National Center for Therapeutics Manufacturing (NCTM) at Texas A&M University, she immediately jumped at the opportunity and applied for a scholarship. |
Upon completion of the program, Nixon was hired at Lonza as a manufacturing technician. Nixon reports that the training she received at NCTM provided a foundation to understand the big picture — where it came from and where it is going. She feels that the program is not only rewarding, but lucrative. “In one month, I was marketable!” Nixon’s supervisor finds tremendous comfort in the fact that Tanya brought to the company the necessary skills to get the job done; and the biopharmaceutical industry is ready for more employees just like her. |
Nixon is convinced that the principles learned during her military service — including attention to detail, timeliness, and integrity — are equally important in the biomanufacturing field. “Soldiers are perfect candidates for [the biomanufacturing] field because they are keenly skilled at thinking on their toes and are very flexible,” Nixon said. |
Through the military workforce training program, NCTM is building upon veterans’ strong sense of responsibility, professionalism, and dedication to accomplishing a mission as well as their ability to work in challenging and demanding environments — all characteristics that make them ideal additions to the biotech industry. MVMV allows us to train former service men and women to protect our country in a new way as biomanufacturing technicians and process development and quality specialists in facilities that are the nation’s first line of defense from biological threats.
Participants in this initiative receive training in the curriculum of NCTM’s biomanufacturing technical certificate (BTC), a program that comprehensively covers both theory and application of CGMPs within the pharmaceutical industry, giving new hires a running start and greatly reducing the need for on-the-job training. The multidisciplinary curriculum offers an in-depth study of upstream and downstream manufacturing processes, sterile environment protocols, bioprocess equipment operations, quality unit operations, documentation practices, and SOPs most common in the pharmaceutical industry. Specific courses include the following:
Introduction to Therapeutics Manufacturing (online course providing fundamentals of development and manufacture of regulated pharmaceutical products)
Understanding the Science of the Biotherapeutics Industry (online course module introducing the scientific principles of microbiology, immunology, and basic biochemistry for developing and manufacturing biopharmaceutical products)
CGMP Procedures and Documentation (online course module covering all proper documentation practices in regulated drug manufacturing environments)
Pharmaceutical Facility Operations (online course module providing instruction on pharmaceutical facility design, clean area design and operation, and sanitization and sterilization)
Safety in the Pharmaceutical Industry (online course module introducing pharmaceutical production workplace safety, industrial hygiene, risk assessment, and basic laboratory safety)
Upstream Manufacturing of Biologics (week-long, hands-on training in upstream bioprocess equipment operations, including production of biomass with various platforms, cell line creation and maintenance, bioreactor preparation and assembly, software tutorials, and analytics used to evaluate upstream production)
Downstream Manufacturing of Biologics (week-long, hands-ontraining in downstream bioprocess equipment operations, including buffer preparation, sterile filtration, cell lysis, centrifugation, product clarification, tangential flow filtration, Unicorn chromatography software training, column packing, ion exchange, and hydrophobic-interaction chromatography).
Quality Unit Operations (week-long, hands-on training in quality assurance systems and practices, quality control activities, and quality control assays that are relevant to biomanufacturing, including environmental monitoring and microbial testing, bacterial endotoxin testing, SDS‐PAGE assay, total protein assay, and HPLC).
Through NCTM-provided leads, upon completion many graduates applied for jobs and/or had interviews with Pfizer, Alcon-Novartis, Lonza, and Fujifilm Diosynth Biotechnologies. The MVMV program was made possible by a Wagner-Peyser grant received from the Governor of Texas’s office and administered through the Texas Workforce Commission. NCTM is currently seeking additional funding sources to continue this program.
The Singapore Institute of Technology’s Pharmaceutical Engineering Program
Susanna Leong and Lim Kok Hwa
Pharmaceutical manufacturing is one of the major pillars of Singapore’s economy. The city-state is a top manufacturing site for active pharmaceutical ingredients and solid dosage drugs for the global market today. In the past five years, pharmaceutical giants have invested significantly in Singapore, culminating in nine world-class biologics manufacturing plants.
To support and sustain pharmaceutical manufacturing activities over the long term, it is critical to train skilled manpower for the sector. The Singapore Institute of Technology (SIT) launched the bachelor of engineering (honors) Pharmaceutical Engineering (PharmE) degree program to fulfill industry employment needs by tapping into Singapore’s undergraduate talents. The goal is to build a critical mass of key employee capabilities that will underpin the long-term growth of the country’s pharmaceutical manufacturing industry and activities. This is the first PharmE program in Singapore and the region, where it is envisaged that a career in the knowledge-intensive pharmaceutical sector would be a good fit with the educational and career aspirations of the Singapore population.
The PharmE program focuses on training students in development and manufacturing of biologics and small-molecule drugs through an interdisciplinary curriculum that combines engineering and science. The instruction seeks to provide students with strong grounding in both engineering and science across the full spectrum of skillsets that are pertinent to drug manufacturing, ranging from drug development and production to process development, operations, validation, regulation, and project management. The program is further distinguished by a curriculum that is strongly girded with cuttingedge industry-compliant concepts, regulations, and know-how. The curriculum was developed in close consultation with industry to align the degree program with industry needs. Industry practitioners also will contribute to the delivery of selected modules to engage students in real industrial case studies.
During their trimester break in the second year of the program, students can choose to undertake an immersion program to gain exposure to industry-like pharmaceutical manufacturing practices, either locally or overseas. The immersion can take the form of visits to GMP drug manufacturing facilities, short-term employment in pharmaceutical companies, or participation in workshops or courses that provide hands-on training in drug manufacturing unit operations at laboratory and pilot scales. For example, to complement bench-scale laboratory skillsets, SIT is working with BTEC to customize a training program focused on fermentation operations in CGMP pilot-scale labs.
Another key feature is the integrated work-study program (IWSP) to provide industry skillsets and enable students to apply taught theory to troubleshoot industry problems. IWSP is an uninterrupted work placement program that each student undergoes as a graduation requirement. Students intern with pharmaceutical companies for a full eight months, giving them the opportunity to acquire in-depth knowledge in industrial operations and hands-on skillsets to complement classroom theory. In the second half of their IWSP, students are tasked to design and complete a capstone project that is centered on solving real industry problems. An extended industrial attachment program will facilitate a structured work-study program for which companies can justify training our students to participate in “real work” in the company. That gives them the chance to apply classroom-acquired theory to troubleshoot problems encountered in their company-assigned project.
The program’s applied learning emphasis and strong partnership with industry are aimed at enhancing our students’ industry readiness and preparing them to hit the ground running when they enter the industry as pharmaceutical engineers. The generation of such a pipeline of highly skilled specialists will be critical to support and sustain the growth of Singapore’s pharmaceutical manufacturing sector.
References
1 Webster’s Third New International Dictionary (unabridged). Gove PB, Editor. Merriam-Webster Inc., Springfield, MA, 1993.
2 NC State University. Master of Microbiol Biotechnology: Industry Practicum; http://harvest.cals.ncsu.edu/master-of-microbial-biotechnology/industry/industry-practicum.
Patty Brown is a senior instructional designer, and Rick Lawless is the associate director of strategic programs, both at BTEC; Mike Fino is the dean of mathematics and sciences at MiraCosta College, north San Diego area, CA ). Jim DeKloe is a professor of biology and biotechnology at Solano Collage, South San Francisco, CA). Jason M. Cramer is the coordinator, and Paul T. Hamilton is the director of the Microbial Biotechnology Program at NCSU. Jenny Ligon is executive director of workforce development at the Texas A&M Center for Innovation in Advanced Development and Manufacturing (College Station, TX). Susanna Leong is an associate professor and cluster director, chemical and engineering and food technology, at the Singapore Institute of Technology , and Lim Kok Hwa is an associate professor at the Singapore Institute of Technology. S. Anne Montgomery is cofounder and editor in chief of BioProcess International. Please direct inquiries to John Balchunas, [email protected].
Patty Brown, Rick Lawless, Mike Fino, Jim DeKloe, Jason M. Cramer, Paul T Hamilton, Jenny Ligon, Susanna Leong, and Lim Kok Hwa, with S Anne Montgomery
You May Also Like





